Osteosarcoma
Osteosarcoma: A Comprehensive Guide to the Primary Bone Cancer
Introduction: The Tumor of Growing Bone
Osteosarcoma is the most common primary malignant bone tumor, a relentless cancer that arises from osteoblasts, the cells responsible for forming new bone. Characteristically aggressive, it produces a chaotic, immature, and weak “osteoid” matrix. While rare in the general population (~1,000 new cases per year in the US), it is a defining disease of adolescence, with a pronounced peak incidence during the pubertal growth spurt (ages 10-14). This timing is not coincidental; it implicates rapid bone proliferation as a key etiological factor. Osteosarcoma presents a formidable challenge due to its propensity for local destruction and early micrometastatic spread, primarily to the lungs. However, the advent of multi-agent chemotherapy combined with limb-salvage surgery has transformed a once nearly universally fatal diagnosis into one where approximately 70% of patients with localized disease achieve long-term survival. This guide details the biology, presentation, multidisciplinary management, and evolving frontiers in the treatment of this complex sarcoma.
Part 1: Biology and Epidemiology – The “Where” and “Who”
Epidemiology:
Bimodal Age Distribution:
Primary Peak: Adolescents and young adults (75% of cases occur in patients under 25). This correlates with periods of maximal skeletal growth.
Secondary Peak: Adults over 60, often associated with Paget’s disease of bone or a history of prior radiation to bone (radiation-induced osteosarcoma).
Sites of Predilection:
Osteosarcoma favors the metaphyseal region (the growing end) of long bones.
Most Common Sites: Distal femur (~45%), proximal tibia (~20%), and proximal humerus (~10%).
“Classic” Presentation: A painful, enlarging mass just above or below the knee.
Risk Factors:
Genetic Syndromes: Patients with hereditary retinoblastoma (germline RB1 mutation) have a >500-fold increased risk. Li-Fraumeni syndrome (TP53 mutation) also confers a high risk.
Benign Bone Conditions: Paget’s disease, fibrous dysplasia.
Prior Radiation Exposure: To a bone field, often with a latency period of 5+ years.
Part 2: Clinical Presentation and the Urgency of Diagnosis
The symptoms, while sometimes mistaken for growing pains or sports injuries, are progressive and unrelenting.
Classic Triad of Symptoms:
Pain: The most common initial symptom. It is deep, aching, and progressive, often worse at night or with activity. It does not resolve with rest.
Swelling/Mass: Becomes apparent weeks to months after pain onset. The mass is firm, fixed to bone, and may feel warm due to increased vascularity.
Loss of Function/Limping: Due to pain or mechanical interference with a joint.
“Red Flag” Signs:
Pain that awakens a child from sleep.
Pain that persists beyond 2-3 weeks, especially if not associated with a clear traumatic event.
A palpable, firm mass associated with bone pain.
Pathologic fracture (a break through a bone weakened by tumor).
Critical Note: Any adolescent with persistent, deep bone pain warrants a thorough orthopedic evaluation and plain X-rays. Early diagnosis is paramount for limb salvage and cure.
Part 3: The Diagnostic Pathway – Imaging and Biopsy
Diagnosis follows a strict sequence to prevent tumor contamination and ensure accurate staging.
Step 1: Plain Radiographs (X-rays) – The First and Most Important Test
Findings are often characteristic:
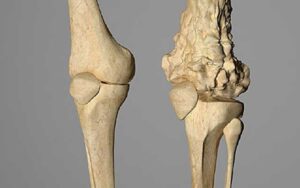
Mixed Lytic and Sclerotic Appearance: The tumor both destroys bone and produces chaotic new bone.
“Sunburst” or “Hair-on-End” Periosteal Reaction: Spicules of new bone forming perpendicular to the cortex.
Codman’s Triangle: An elevated periosteum where it is lifted off the bone by the advancing tumor.
Permeative Destruction: Poorly defined transition zone between tumor and normal bone.
Step 2: Cross-Sectional Imaging for Local Staging
MRI of the Entire Involved Bone: The gold standard. Defines the full intramedullary (marrow) extent of the tumor, soft tissue mass, relationship to neurovascular structures, and skip metastases (tumor foci within the same bone). Essential for surgical planning.
CT Scan of the Chest: To detect lung metastases, the most common site of spread.
Step 3: Biopsy – The Definitive Diagnostic Step
Must be performed by or in consultation with the surgical oncologist who will perform the definitive resection. An incorrectly placed biopsy tract can compromise limb-salvage options.
Core needle biopsy is preferred, providing adequate tissue for histology and molecular studies.
Histology shows pleomorphic, malignant spindle cells producing osteoid (pink, amorphous extracellular material).
Step 4: Systemic Staging & Baseline Studies
Whole-Body Bone Scan (Tc-99m MDP): To evaluate for other bony metastases.
PET/CT: Increasingly used to assess metabolic activity and detect distant metastases.
Blood Tests: Elevated Alkaline Phosphatase (ALP) and Lactate Dehydrogenase (LDH) are poor prognostic markers.
Part 4: Multimodal Treatment – The Cornerstone of Cure
Treatment for high-grade osteosarcoma is never surgery alone. The current paradigm is neoadjuvant chemotherapy → surgery → adjuvant chemotherapy.
Phase 1: Neoadjuvant (Preoperative) Chemotherapy (Approx. 10 weeks)
Goals:
Treat micrometastatic disease immediately (present in 80%+ of patients at diagnosis, even if not visible on scans).
Shrink the primary tumor, making limb-salvage surgery easier and safer.
Assess tumor response (histologic necrosis), which is a powerful prognostic factor.
Drugs: High-dose, multi-agent regimens. The classic backbone includes:
Doxorubicin (Adriamycin)
Cisplatin
High-Dose Methotrexate (with leucovorin rescue)
Ifosfamide and Etoposide are often added for poor-risk or metastatic disease.
Phase 2: Definitive Surgical Resection
The goal is wide surgical margins—removing the tumor with a complete cuff of normal tissue.
Limb-Salvage Surgery (LSS): The standard of care in >90% of cases. The tumor-bearing bone is removed and replaced with:
Metallic Endoprosthesis (expandable in growing children).
Allograft (cadaver bone).
Rotationplasty: A functional, life-saving procedure for tumors around the knee where the ankle becomes the knee joint, fitted with a below-knee prosthesis. Offers excellent mobility.
Amputation: Required if the tumor involves major neurovascular structures or is not amenable to LSS. Modern prosthetics allow for excellent function.
Pathological Assessment: The resected tumor is analyzed to determine the percent tumor necrosis from chemo. >90% necrosis (“good responder”) correlates with significantly better survival.
Phase 3: Adjuvant (Postoperative) Chemotherapy
Continues for several months based on the original regimen and the tumor’s response. Poor responders may have their chemotherapy regimen intensified.
Treatment of Metastatic Disease
Pulmonary metastases are surgically resected (metastasectomy) if possible.
More intensive chemotherapy and novel agents are used. Long-term survival for metastatic disease remains poor (~30%).
Part 5: Prognosis, Survivorship, and Future Directions
Prognostic Factors:
Favorable: Localized disease, good histologic response to chemo (>90% necrosis), tumors of the extremities (vs. axial skeleton).
Unfavorable: Presence of metastases at diagnosis, poor histologic response, axial (pelvic, spine) location, large tumor size, elevated ALP/LDH.
Survivorship Challenges:
Cure comes at a cost. Survivors face lifelong issues:
Limb Function: Complications of prostheses, allografts, or amputations.
Cardiotoxicity: From doxorubicin.
Renal Toxicity & Hearing Loss: From cisplatin.
Secondary Malignancies: Risk from chemotherapy and radiation.
Psychosocial Impact: Requiring long-term monitoring and support.
Emerging Therapies and Research:
Immunotherapy: Checkpoint inhibitors (e.g., pembrolizumab) and CAR T-cells targeting specific antigens are in early-phase trials.
Targeted Therapies: Based on genomic profiling (e.g., CDK4 amplification, MDM2 amplification). Drugs like Pazopanib (a multi-kinase inhibitor) and Denosumab (for giant cell-rich subtypes) are being studied.
Lung Metastasis Therapy: Inhalational chemotherapy (e.g., cisplatin) is under investigation.
Conclusion: A Battle Fought on Multiple Fronts
Osteosarcoma represents a paradigm of multimodal cancer therapy, where the integration of systemic chemotherapy with radical, precise surgery has turned the tide. Its predilection for the young adds a profound urgency to the pursuit of both cure and quality of life. While challenges remain—particularly for metastatic and poor-responder disease—the field is moving beyond traditional chemotherapy. Through international clinical trials, molecular profiling, and advances in surgical reconstruction, the goal is to continue improving survival rates while minimizing lifelong morbidity for young survivors. For patients and families, the journey is arduous, but it is navigated within a framework of established protocols and growing hope fueled by relentless research.